Dear readers, the following article is about the chemistry of atomic particles, which are the main constituents of the universe. I hope you will find it useful. It will help you to understand Zia God energy theory and the role of atomic particles in different interactions.
Atoms are the basic particles of chemical elements. All atoms of an element are the same, but different from the atoms of another element. All atoms are composed of nucleus contains protons and neutrons and the electrons revolving around the nucleus. All atoms are electrically neutral, because every atom has an equal number of negatively charged electrons and positively charged protons. Their charges are equal in magnitude.
- Matter is composed of atoms proposed by john Dalton in (1808),
- Electrons discovered by JJ Thomson in (1897),
- Protons discovered by E. Rutherford in (1911).
- Neutron discovered by James Chadwick in (1932)
- Atomic model provided by Bohr in (1940)
Science reveals the secrets of nature !
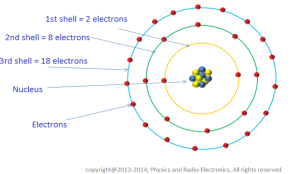
All atoms have orbits or shells (1,2 3,4…) or (K L M N O..) sub- shells (s, p, d, f) and orbitals (px, py, pz) in the sub- shells around the nucleus in which the electrons retain with certain rules like:
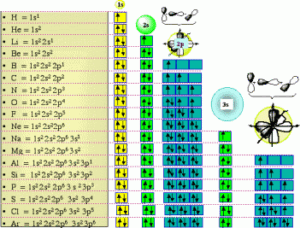
1. Electrons tend to occupy first the lowest energy levels available. 2. The 1st shell contains 2 electrons, the 2nd shell 8 electrons, the 3rd shell 18 electrons and so on by formula (2 x n2) where n is the number of shells. 3. An orbital can contain a maximum of two electrons with opposite spins. 4. Electrons prefer parallel spin in separate orbitals of sub-shells.
Shape of orbital
An orbital is a region in space where electrons are found. The orbitals have different shapes.
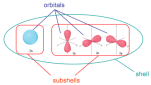
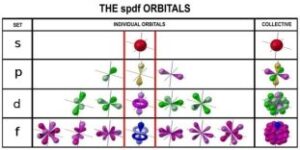
The s orbitals in s sub-shell are spherical, orbitals, in p sub-shell are two-lobed, orbitals, in d sub-shells are four-lobed and the orbitals in the f sub-shell are more complex, so leave it for the time being.
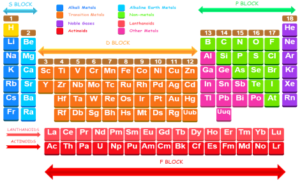
Division of elements
The elements in the periodic table have been divided in S, P, D, and F blocks elements on the basis of electronic configurations of their atoms as shown:
Electronic Configuration

1st K shell consists of only one sub shell (1s), 2nd L shell consists has two sub shells, (2s), (2p), 3rd M shell consists of three sub shells, (3s) (3p) (3d), 4th N shell consists of 4 sub shells (4s) (4p) (4d) (4f). Only two electrons are possible per orbital. (s) orbitals have only two electrons with opposite spins in it. p orbitals have further three sets, each with two electrons, i.e., px, py and pz ). The s, p, d, and f stand for sharp, principal, diffuse and fundamental. Names given to orbitals on the basis of spectral lines).
Filling order of electrons in the orbitals
The Aufbau principle says that electrons will fill up the (lowest energy level) first as follows,
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d10 7 p6
Electronic configuration of some elements >

Quantum numbers
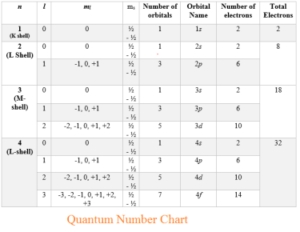
There are four quantum numbers:
1. Principal quantum number (n) describes the electron’s energy level. Its values are 1, 2, 3,4
2. Angular quantum number (ℓ) describes the electron’s orbital. Its value are from 0 to (n-1)
3. Magnetic quantum number (mℓ) describes the orientation of the orbital with values ranging from -ℓ to ℓ.
4. Spin quantum number (ms) describes the spin with value either -1/2 or +1/2
According to the Pauli Exclusion Principle, no two electrons in an atom can have the same values of four quantum numbers.
Chemical Bonding
Atoms bind themselves in different ways. Three ways are as follows:
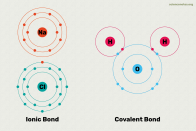
1. Ionic bond: It is formed when an atom donates outermost electrons to another atom like sodium chloride (NaCl)
2. Covalent bond: is formed when electrons are shared between two atoms, like H2O
3. Coordinate covalent bond: A coordinate bond is a covalent bond in which both electrons or a pair of electrons come from the same atom.
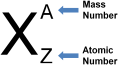
Mass number (A) Mass number is the total number of protons and neutrons present in the nucleus. Atomic number (Z) No. of protons in the nucleus of an atom. No of neutron = (A – Z)
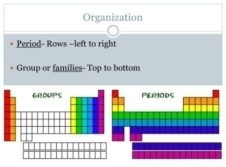
Groups: There are 18 groups top to bottom in the periodic table. Elements in a group have similar but not identical properties. All elements in a group have the same number of electrons in the outer orbital.
Periods: There are 7 periods, left to right across the periodic table. Elements in periods are not alike in properties. The properties change greatly across the periods.
Note: Metals have 1, 2, or 3 valence or outermost electrons while non- metals have 4, 5, 6, or 7 valence electrons.Metals have the tendency to lose electrons, while non -metals have the tendency to gain electrons.
Let us see the trend of behavior of elements in period -1 and period – 2 of the periodic table.
1st Period
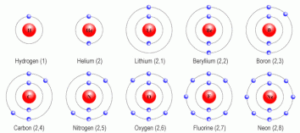
1. Hydrogen has only one shell and has 1 electron in its only shell. Hydrogen atom requires one more electron to achieve the noble gas most stable configuration therefore reacts vigorously with another hydrogen atom to form hydrogen gas. It is a lighter and flammable gas.
2. Helium has 2 electrons in its only shell and is complete. Therefore inactive and inert gas.
2nd period
Note: All elements in the 2nd period have 2 shells K and L. K can accommodate 2 and L shell 8 electrons in it.
3. Lithium is a very soft light alkali metal. Lithium has one electron at its outermost shell. Its unpaired electron is very loosely bound hence very reactive. It is a silvery white metal because it can lose one electron more easily.
4. Beryllium is a relatively soft lightest alkaline earth metal. It has got 2 electrons in its outermost shell, therefore slightly less reactive than lithium.
5. Boron is a metalloid (semi metal). Boron has equal capability of losing as gaining electrons so it behaves as a metalloid. The other elements in the same group have more shells, so it is much easier for them to lose their valence electrons and they behave as metals.
6. Carbon is true non-metal. It has 4 electrons in the valence shell. To satisfy the octet rule it has to gain more 4 electrons which is not possible for 6 protons in the nucleus to hold 10 electrons or to lose 4 electrons which is also not possible because it requires more energy. Therefore the only possible way is to share its 4 electrons and make covalent bonds.
7. Nitrogen is definitely non-metal as it does not readily give up its electrons. Non metals are extremely greedy for electrons and take them from metals. Moreover, it is a poor conductor of heat and electricity. It is colorless and odorless gas.
8. Oxygen has 6 electrons in the outermost shell therefore has high affinity for electrons. Oxygen tends to take in two electrons, and become a negative ion whereas metals tend to give up electrons and become positive ions. Oxygen can also form covalent bonds, which metals do not. It is a combustible gas.
9. Fluorine has 7 outermost electrons; hence it prefers to gain one more electron to attain inert or noble gas configuration or obey octet rule. It is therefore the most reactive element in its group. It is a pale yellow toxic gas
10. Neon is an inert gas. All noble gasses are non-metals. Since non-metal is the element that accepts an electron to complete their octet while noble gasses already having a complete octet. Its valence shell is completely filled, so it is chemically not reactive. It is an inert gas.
Atomic radii decreases in periods because of the same numbers of shells and the electrons and protons are close to each other, whereas it increases in groups because the number of shells increases and the attraction between electrons and protons decreases.
Electron affinity increases in periods because of the same number of shells and electrons and protons are close to each other, whereas it decreases in groups because of more number of shells and electrons and protons are farther from each other.
Transition elements: They exhibit magnetic properties, have variable oxidation states and their compounds are colored etc.
Outer transition elements: They are d block elements with incompletely filled d orbitals.
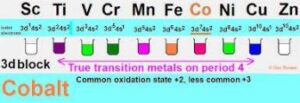
Inner transition elements: They are f block elements with filled or partially filled 4f and 5f orbitals.
Lanthanides: Elements between Z (57 -71) are called lanthanides. Their valence electrons are in 4 f orbitals. Promethium is radioactive, the rest are not. Oxidation states from +3 to +4. They do not form complexes.
Actinides: Elements between Z (90 -103) are called actinides. Their valence electrons are in 5 f orbitals. All are radioactive elements. Oxidation states from to +3 to +6. They form complexes.
Metal Complexes: A metal complex consists of a central metal atom or ion that is bonded to one or more ligands. Ligand is a molecule or ion that forms a co-ordinate bond with transition metal by donating a pair of electrons.
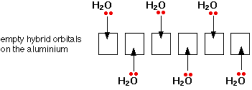
Example: Aluminum water complex ion, also known as the hexa-aqua aluminum(III) ion, is an example. [Al(H₂O)₆]³⁺
Electronic configuration of Aluminum :1s2 2s2 2p6 3s2 3p1
Aluminum re-organizes 3s ,all the 3p and two of the 3d orbitals to produce 6 new orbitals all with the same energy for six water molecules that can fit around the aluminum ion.
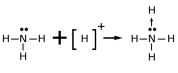
Atoms tend to combine in such a way that each atom acquires two electrons in their valence or outmost shell Duet rule or eight electrons in its valence shell Octet rule (stable electronic configuration)
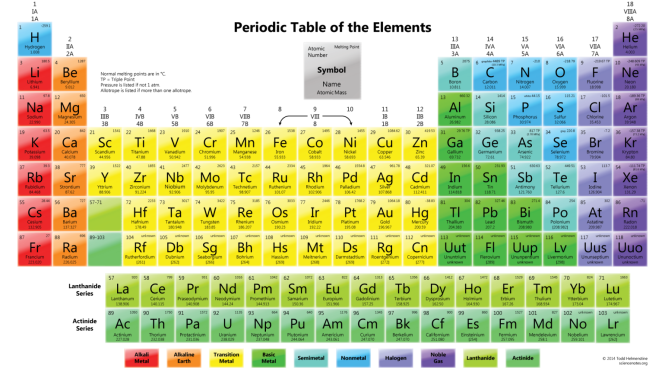
Periodic Table of elements: Look how, how all the elements are arranged in the periodic table in the ascending order of their atomic numbers. All the elements discovered so far are about (118 ). Nothing missing in the periodic table. Some are not shown in the following Mendeleev periodic table. You can check other periodic tables for 118 elements.
Q.How did it happen so perfectly?
Zia Ahmed Khan e-mail : khanziaahmed50@gmail.com